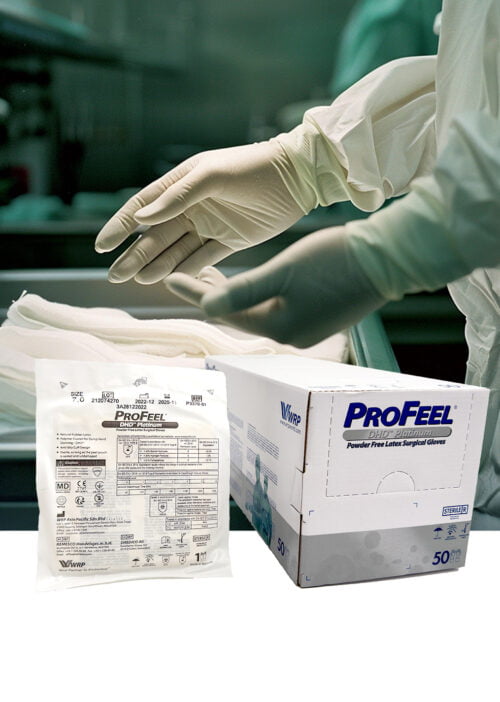
PROFEEL® DHD™ DOUBLE GLOVING POLYISOPRENE PF Surgical Gloves: Enhanced Protection and Comfort
Experience the ultimate level of protection and comfort with PROFEEL® DHD™ DOUBLE GLOVING POLYISOPRENE PF Surgical Gloves. This sterile double-gloving system comes with two pairs of gloves per pack: an inner glove and an overglove, both crafted from comfortable and flexible polyisoprene.
Key features and benefits:
- Double the protection: Wearing two pairs of gloves significantly reduces the risk of contamination by providing an additional barrier and minimizing perforations to the inner glove.
- Exceptional comfort: The polyisoprene material delivers a soft, flexible fit that conforms to your hand, reducing fatigue even during extended procedures.
- Enhanced grip and control: The micro-roughened surface ensures a secure grip on instruments, both wet and dry, allowing for precise control and optimal tactile sensitivity.
- Easy donning: The DHD™ (Damp-Hand-Donning) technology facilitates comfortable donning even with damp hands.
- Reliable protection: The gloves are chemotherapy-tested and feature a beaded cuff that creates a secure seal with your gown, minimizing the risk of contamination.
- Easy identification: The cuff is clearly printed with the size and logo for quick and easy identification.
PROFEEL® DHD™ DOUBLE GLOVING POLYISOPRENE PF Surgical Gloves are the perfect choice for healthcare professionals who demand the highest level of protection and comfort in high-risk procedures or situations where additional barrier protection is required.
Medical Device – Class IIa
Class IIa devices are subject to specific regulatory requirements, such as premarket review by a national regulatory agency, such as the FDA in the US, and MDR in the EU. Manufacturers of Class IIa devices must also comply with general safety and performance standards and must provide clinical data to demonstrate the safety and effectiveness of the device.
ISO 9001:2015 – Certified
We are a Denmark-based ISO 9001-certified medical device manufacturer, importer, and distributor. By following a strict QMS, we ensure that our clients always get the highest attention and the best quality products.

REACH
The REACH Regulation was introduced by the EU in 2007. Its aim is to improve the protection of people and the environment from the risks posed by chemicals. REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemicals.
Quality Standards
-
EN 455-1:2020, EN 455-2015, EN 455-3:2015, EN 455-4:2009
EN 374-1:2016, EN 374-1:2016/A1:2018, EN 374-2:2019
EN 374-4:2019, EN 374-5 :2016. EN 16523-1:2015+A12018, EN420:2003+A1:2009 -
ISO 9001:2015, ISO 13485:2016, ISO 14001:2015
-
Medical Device, Class IIa, in accordance to MDR 2017/745
-
Personal protective equipment in accordance to Regulation (EU) 2016/425
CE Category III: For irreversible or mortal risks
Specifications
| Thickness (mm) | Specification |
|---|---|
| Finger | 0.21 ± 0.03 |
| Physical Properties | Unaged | Aged |
|---|---|---|
| Tensile Strength (MPa) | Min. 24 | Min. 18 |
| Ultimate Elongation (%) | Min. 750 | Min. 560 |
| Stress at 500% (MPa) | Max. 5.5 | N/A |
| Force at break (N) | > 9 | > 9 |