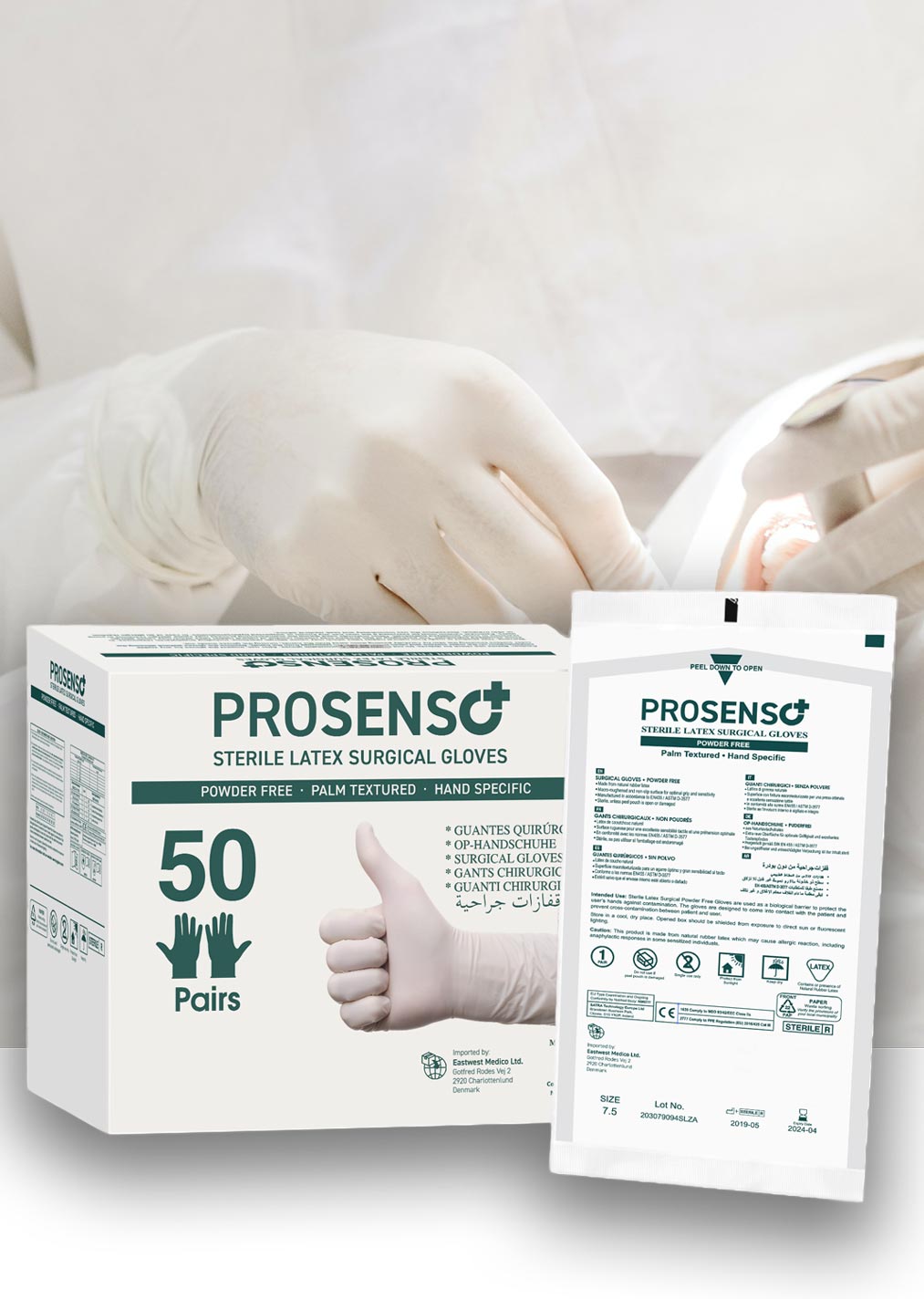
PROSENSO™ SURGICAL
Introducing PROSENSO™ SURGICAL Latex Gloves. The perfect solution for any surgeon who needs the highest level of quality and sterility. Our gloves are designed to provide superior protection and performance and are made from premium latex material for a reliable fit and feel. They provide superior strength and durability and feature an ergonomic design that ensures comfort and dexterity. Our PROSENSO™ SURGICAL Latex Gloves are the perfect choice for any surgical procedure, offering superior protection, reliability, and comfort.
Premium Latex – Professional Series
Premium latex is a high-quality material used in the production of PROSENSO™ SURGICAL. This type of latex is known for its strength and durability, as well as its resistance to punctures and tears. The material is also highly elastic, which allows the gloves to fit snugly on the hands of the surgeon while still providing maximum dexterity and movement.
Medical Device – Class IIa
Class IIa devices are subject to specific regulatory requirements, such as premarket review by a national regulatory agency, such as the FDA in the US, and MDR in the EU. Manufacturers of Class IIa devices must also comply with general safety and performance standards and must provide clinical data to demonstrate the safety and effectiveness of the device.
ISO 9001:2015 – Certified
We are a Denmark-based ISO 9001-certified medical device manufacturer, importer, and distributor. By following a strict QMS, we ensure that our clients always get the highest attention and the best quality products.

REACH
The REACH Regulation was introduced by the EU in 2007. Its aim is to improve the protection of people and the environment from the risks posed by chemicals. REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemicals.
Quality Standards
-
EN 455-1:2020, EN 455-2015, EN 455-3:2015, EN 455-4:2009
EN 374-1:2016, EN 374-1:2016/A1:2018, EN 374-2:2019
EN 374-4:2019, EN 374-5 :2016. EN 16523-1:2015+A12018, EN420:2003+A1:2009 -
ISO 9001:2015, ISO 13485:2016, ISO 14001:2015
-
Medical Device, Class IIa, in accordance to MDR 2017/745
-
Personal protective equipment in accordance to Regulation (EU) 2016/425
CE Category III: For irreversible or mortal risks
Specifications
- Weight per glove: 10.8 g