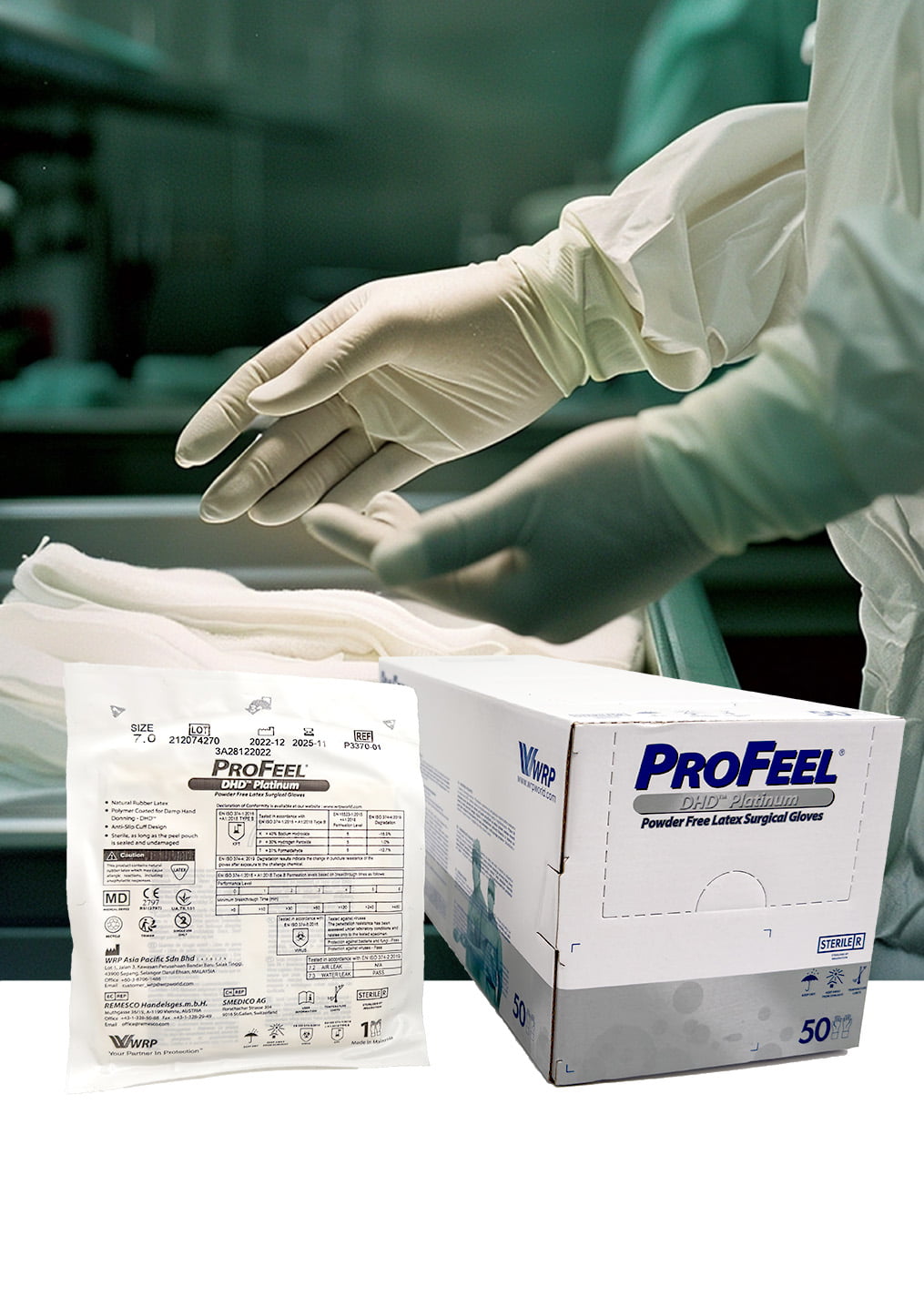
Elevate surgical performance with PROFEEL® DHD™ PLATINUM LATEX PF Surgical Gloves. These sterile, powder-free gloves are meticulously crafted to meet the demanding needs of healthcare professionals, offering a superior combination of comfort, protection, and dexterity.
Key features and benefits:
- Exceptional comfort: Made from natural rubber latex, these gloves provide a soft, flexible fit that reduces hand fatigue during extended procedures.
- Enhanced dexterity: The micro-roughened surface ensures a secure grip on instruments, both wet and dry, allowing for precise control and tactile sensitivity.
- Unmatched protection: The powder-free design minimizes the risk of allergies and irritation, while the beaded cuff creates a secure seal with your gown, reducing the risk of contamination.
- Long-lasting performance: The gloves are polymer coated for easy donning and maintain their softness and elasticity even after extended storage.
PROFEEL® DHD™ PLATINUM LATEX PF Surgical Gloves are the perfect choice for surgeons, nurses, and other healthcare professionals who demand uncompromising quality, comfort, and protection in the operating room.
Medical Device – Class IIa
Class IIa devices are subject to specific regulatory requirements, such as premarket review by a national regulatory agency, such as the FDA in the US, and MDR in the EU. Manufacturers of Class IIa devices must also comply with general safety and performance standards and must provide clinical data to demonstrate the safety and effectiveness of the device.
ISO 9001:2015 – Certified
We are a Denmark-based ISO 9001-certified medical device manufacturer, importer, and distributor. By following a strict QMS, we ensure that our clients always get the highest attention and the best quality products.

REACH
The REACH Regulation was introduced by the EU in 2007. Its aim is to improve the protection of people and the environment from the risks posed by chemicals. REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemicals.
Quality Standards
-
EN 455-1:2020, EN 455-2015, EN 455-3:2015, EN 455-4:2009
EN 374-1:2016, EN 374-1:2016/A1:2018, EN 374-2:2019
EN 374-4:2019, EN 374-5 :2016. EN 16523-1:2015+A12018, EN420:2003+A1:2009 -
ISO 9001:2015, ISO 13485:2016, ISO 14001:2015
-
Medical Device, Class IIa, in accordance to MDR 2017/745
-
Personal protective equipment in accordance to Regulation (EU) 2016/425
CE Category III: For irreversible or mortal risks
Specifications
| Thickness (mm) | Specification |
|---|---|
| Finger | 0.21 ± 0.03 |
| Physical Properties | Unaged | Aged |
|---|---|---|
| Tensile Strength (MPa) | Min. 24 | Min. 18 |
| Ultimate Elongation (%) | Min. 750 | Min. 560 |
| Stress at 500% (MPa) | Max. 5.5 | N/A |
| Force at break (N) | > 9 | > 9 |