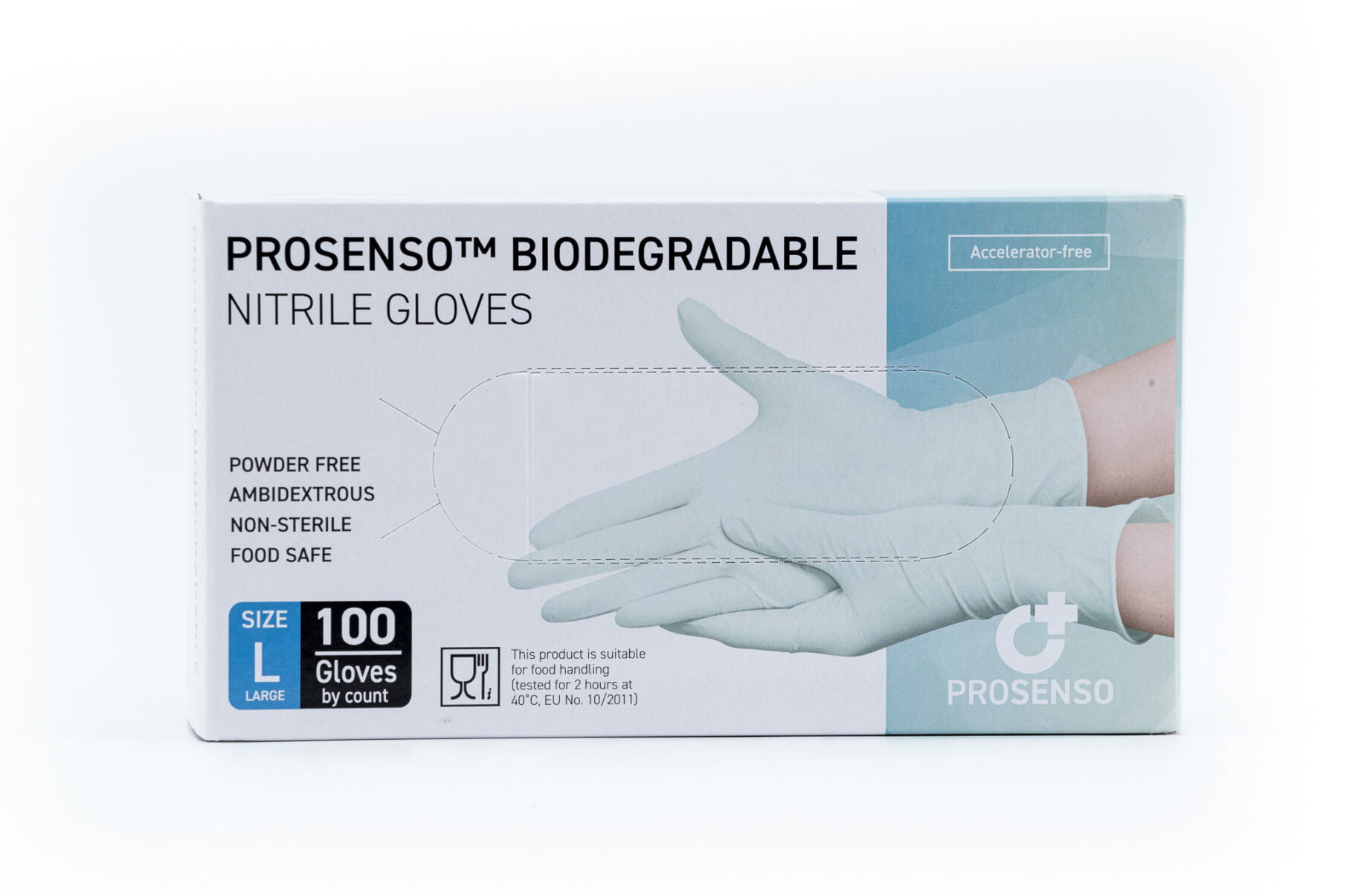
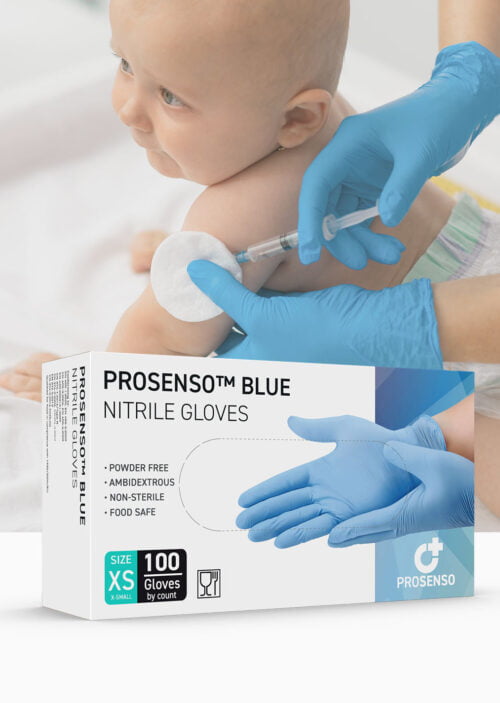
PROSENSO™ BIODEGRADABLE is a groundbreaking nitrile glove manufactured by Eastwest Medico ApS, setting new standards in sustainability and comfort.
Revolutionary Biodegradability:
PROSENSO™ BIODEGRADABLE is the first biodegradable and accelerator-free nitrile glove available in the market. These gloves are made with a unique NBR formula (Nitrile Butadiene Rubber) that enables them to biodegrade through microbial action in both aerobic and anaerobic environments within a remarkable timeframe of 3-5 years. In comparison, regular nitrile gloves can take 100-200 years to biodegrade.
Biodegradation Process:
The biodegradation of PROSENSO™ BIODEGRADABLE occurs in a two-step process. Initially, the glove’s surface undergoes erosion, breaking down the outermost layer. In the second step, microorganisms secrete enzymes that chemically break down the remaining glove material. This process yields a nutrient-rich, water-insoluble food source that the microbial population can readily consume.
Premium Nitrile with Low Modulus:
PROSENSO™ BIODEGRADABLE utilizes a patented premium low-modulus formula, distinguishing it from regular nitrile gloves. This innovative formulation results in a softer and more flexible glove, providing superior comfort and dexterity. Discover the unparalleled combination of biodegradability and premium nitrile performance with PROSENSO™ BIODEGRADABLE.
Accelerator-Free for Maximum Comfort:
To ensure maximum comfort, PROSENSO™ BIODEGRADABLE gloves are entirely manufactured without chemical accelerators. Unlike 95% of nitrile gloves in the market, which may cause Type IV Hypersensitivity with long-term use, these gloves are accelerator-free. Experience optimal skin protection and comfort without compromising performance.
Certified Medical Device and PPE:
PROSENSO™ BIODEGRADABLE gloves hold certifications as a Medical Device Class I and PPE CAT III, meeting stringent quality and safety standards. These gloves have undergone extensive testing against various chemicals, including fentanyl and cytostatic drugs (chemo). They are an ideal choice for high-risk situations where low-weight gloves are preferred, ensuring both the wearer and the patient are protected.
ISO 9001, ISO 13485, and ISO 14001 Certified:
As a Denmark-based medical device manufacturer, importer, and distributor, PROSENSO™ is proud to be ISO 9001, ISO 13485, and ISO 14001 certified. These certifications underscore our commitment to exceptional quality, regulatory compliance, and environmental responsibility. Our strict adherence to a comprehensive Quality Management System (QMS) guarantees the highest attention and the best quality products for our valued clients.

Compliance with REACH Regulations:
PROSENSO™ prioritizes the protection of people and the environment by strictly adhering to the REACH Regulation introduced by the EU in 2007. This regulation aims to enhance chemical risk management and safeguard human health and the ecosystem. Rest assured that PROSENSO™ BIODEGRADABLE gloves meet the stringent safety standards established by REACH.
Embrace sustainability and uncompromising comfort with PROSENSO™ BIODEGRADABLE. Experience the remarkable biodegradability, premium nitrile performance, and accelerator-free comfort of these groundbreaking gloves. Trust in our expertise and join us in reducing environmental impact while ensuring superior hand protection.
Quality Standards
-
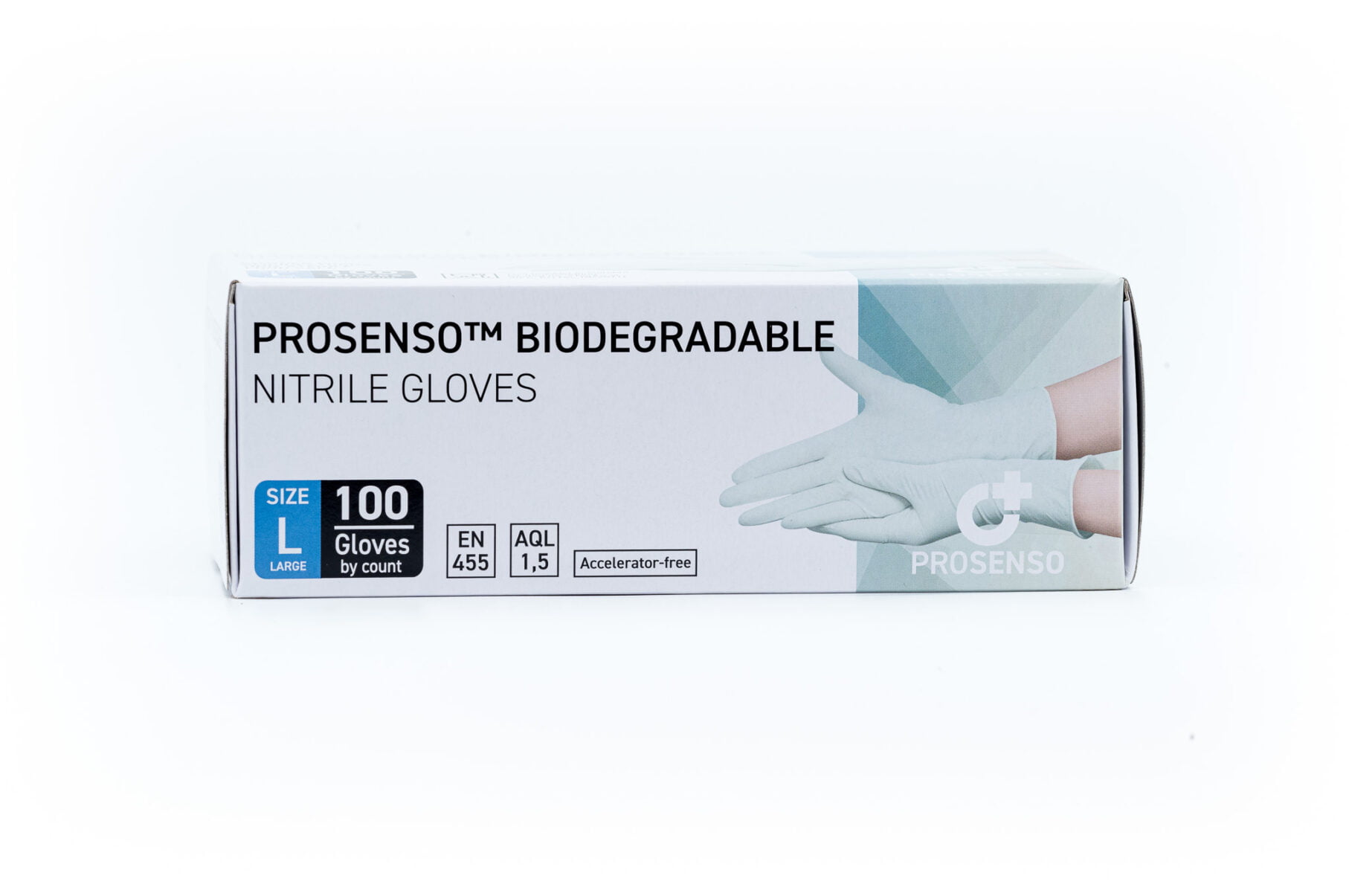
EN 455-1:2020, EN 455-2015, EN 455-3:2015, EN 455-4:2009
EN 374-1:2016, EN 374-1:2016/A1:2018, EN 374-2:2019
EN 374-4:2019, EN 374-5 :2016. EN 16523-1:2015+A12018, EN420:2003+A1:2009 -
ISO 9001:2015, ISO 13485:2016, ISO 14001:2015
-
Suitable for foodstuffs in accordance to Framework Regulation (EC) No 1935/2004
German Food and Feed Code (LFGB)
Recommendation of the German Federal Institute for Risk Assessment (BfR XXI)
Category 3 ‘’For short contact with foodstuffs’’ -
Medical Device, Class 1, in accordance to MDR 2017/745
-
Personal protective equipment in accordance to Regulation (EU) 2016/425
CE Category III: For irreversible or mortal risks
Specifications
- Ambidextrous
- Food Safe
- Powder-Free
- Single-Use Only