Understanding the EN455-2:2015 standard is crucial for manufacturers, distributors, and users of single-use medical gloves. This set of guidelines, ratified by the European Committee for Standardization (CEN), outlines the vital physical property requirements and testing methods to ensure optimal protection against cross-contamination for patients and healthcare professionals.
This article will delve into the specifics of the EN455-2:2015 standard, exploring its various facets, from its scope and normative references to dimensions and strength requirements and, ultimately, its implications on labeling and test reports. If you are looking for a brief non-detailed overview of all 4 chapters of the EN455-standard, we recommend that you look at our introduction to the EN455-standard.
Scope of EN455-2:2015
The EN455-2:2015 standard is designed to stipulate the requirements and testing methods for the physical properties of single-use medical gloves. This includes surgical gloves that are sterile and anatomically shaped and examination or procedure gloves that can be either sterile or non-sterile. The standard aims to ensure that these gloves provide adequate protection from cross-contamination during medical procedures.
Normative References
Several documents are normatively referenced in the EN455-2:2015 standard. These include:
- EN455-4:2009 – Specifications for the shelf life determination of single-use medical gloves.
- EN1041:2008+A1:2013 – Information provided by the manufacturer of medical devices.
- EN ISO 15223-1:2012 – Symbols to be used with medical device labels, labeling, and information to be supplied.
- ISO 188:2007 – Standards for accelerated aging and heat resistance tests of rubber, vulcanized, or thermoplastic.
- ISO 23529:2010 – General procedures for preparing and conditioning test pieces for physical test methods.
Terms and Definitions
For a clear comprehension of the EN455-2:2015 standard, it’s essential to understand specific terms and definitions. These include:
- Medical Gloves for Single Use: Gloves designed for use in the medical field to protect both the patient and user from cross-contamination.
- Surgical Gloves: Sterile, anatomically shaped medical gloves with the thumb positioned towards the palmar surface of the index finger, intended for use in invasive surgery.
- Examination/Procedure Gloves: These can be either sterile or non-sterile and may or may not be anatomically shaped. They are intended for conducting medical examinations, diagnostic and therapeutic procedures, and for handling contaminated medical material.
- Lot: A collection of gloves of the same design, color, shape, size, and formulation, manufactured at essentially the same time, using the same process, raw materials of the same specifications, common equipment, and packed in the same type of individual container. It is important to note that the EN455-2:2015 standard does not dictate the size of a lot, although it does highlight the potential issues associated with the distribution and control of larger lots. A recommended maximum individual lot size is 500,000 units.
Dimensions of Gloves
The dimensions of the gloves, as specified by the EN455-2:2015 standard, are crucial to consider in any production or distribution process. The dimensions include the gloves’ length and width, and specific measurement procedures are outlined for each.
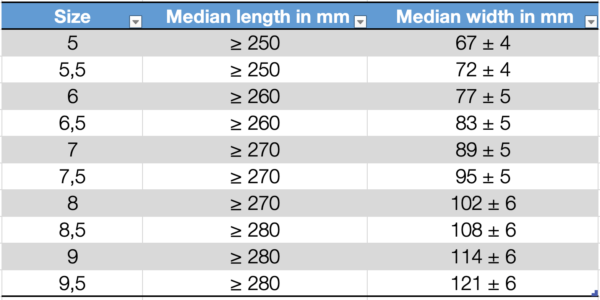
Dimensions of surgical gloves
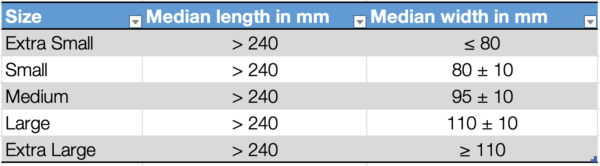
Dimensions of examination/procedure gloves
Length Measurement
The length of the glove is measured by freely suspending the glove with the middle finger on a vertical graduated rule having a rounded tip to fit the shape of the fingertip of the glove. Wrinkles and folds should be removed without stretching the glove, and the median measured length should be recorded.
Width Measurement
The width of the glove is measured using a ruler, with the glove placed on a flat surface. The glove should not be stretched during this process.
The standard provides defined median values for the length and width of surgical gloves and examination/procedure gloves.
Strength Requirements
The EN455-2:2015 standard also outlines the strength requirements for medical gloves. It acknowledges that different glove materials require different force at break requirements to ensure acceptable performance. The standard provides specific force at break values for surgical gloves and examination/procedure gloves, and also lays out the process for testing the force at break after challenge testing.

Test Reports
The standard also stipulates the information that should be included in any test report. This includes an explicit reference to the EN455-2:2015 standard, the type of glove and the manufacturing batch code, the name and address of the manufacturer or distributor, the date of testing performed, and the test results.
Labeling Requirements
Finally, the EN455-2:2015 standard demands specific labeling requirements to be adhered to. These requirements align with the EN ISO 15223-1:2012 and EN 1041:2008+A1:2013 standards. The date of manufacture, defined as the packaging date, must be indicated on the glove and/or the packaging.
Conclusion
In summary, the EN455-2:2015 standard provides a comprehensive set of guidelines for the production and distribution of single-use medical gloves. By adhering to these standards, manufacturers and distributors can ensure the quality and safety of their products, ultimately protecting both healthcare professionals and patients from the risk of cross-contamination.
With a thorough understanding of the EN455-2:2015 standard, stakeholders in the healthcare industry can make more informed decisions, reinforcing the safety and efficacy of their medical gloves and contributing to improved patient outcomes. This understanding also empowers consumers, providing them with the knowledge needed to make informed choices when selecting single-use medical gloves.
In you are interested in studying the standard in details it can be purchased from BSI on this link: https://knowledge.bsigroup.com/products/medical-gloves-for-single-use-requirements-and-testing-for-physical-properties
