
In medical safety, the EN455-1 standard holds a pivotal position. It is a stringent regulation that sets forth the requirements and testing method for medical gloves for single-use to ensure freedom from holes, thereby reducing the risk of cross-contamination during medical procedures. This article is an extension to our article Understanding EN455: An introduction to the Medical Glove Standard and delves into the intricacies of the EN455-1:2020 standard, providing an in-depth understanding of its purpose, structure, and implications for the medical field.
1. The Essence of EN455-1:2020+A1:2020
The EN455-1 standard is the first part of a suite of regulations that govern the manufacturing and testing of medical gloves for single use. It focuses on ensuring that gloves do not leak, thereby enhancing their effectiveness in preventing cross-contamination.
1.1. What EN455-1 Regulates
The EN455-1 standard specifically stipulates that medical gloves for single use should not leak when subjected to the water tightness test. This test is crucial as it checks for holes in the gloves, which, if present, could compromise their protective function.
1.2. The Role of EN455-1
The role of the EN455-1 standard is to safeguard both the user and the patient from cross-contamination during a single procedure. It does so by setting a high bar for the quality of medical gloves, thereby ensuring their reliability and effectiveness.
2. Components of EN455-1
The EN455-1 standard is made up of several key components, each of which plays a specific role in establishing the requirements for medical gloves for single use.
2.1. Normative References
The EN455-1 standard does not rely on any normative references. This means that it stands independently and does not need to refer to or borrow from any other standards to define its terms or set its requirements.
2.2. Terms and Definitions
The EN455-1 standard incorporates specific terms and definitions essential for its application. For instance, it defines medical gloves for single use as gloves intended for use in the medical field to protect the patient and user from cross-contamination during a single procedure. It also defines a hole as a defect in the glove that allows for water leakage and a lot as a collection of gloves of the same design and specifications.
2.3. Water Tightness Test
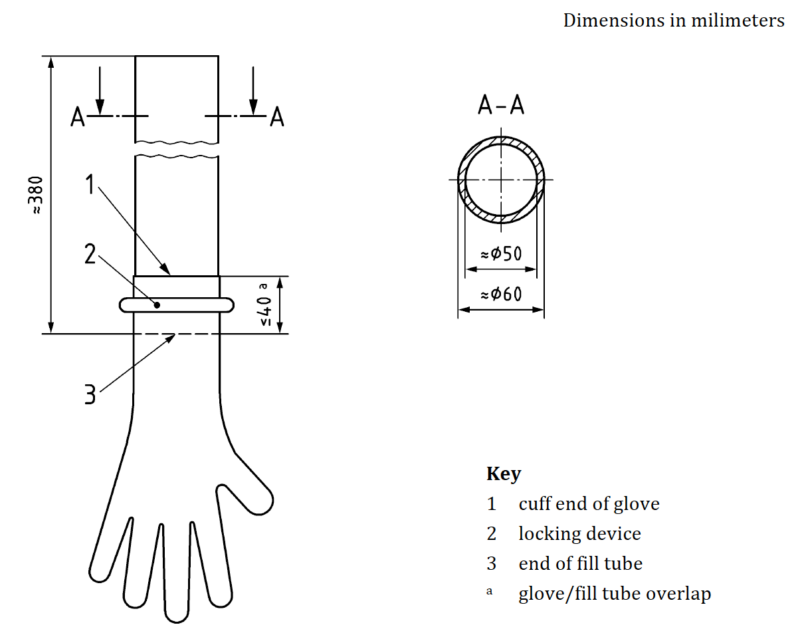
A significant part of the EN455-1 standard is the water tightness test, which is used to detect the presence of holes in the gloves. The standard provides a detailed methodology for conducting this test, including the use of a filling tube of suitable dimensions and the addition of a specific amount of water at a certain temperature.
2.4. Sampling and Inspection Level
The EN455-1 standard also includes guidelines on sampling and inspection levels. It prescribes using standardized Acceptance Quality Limit (AQL) sampling procedures for inspection. For the testing of freedom from holes, the standard sets an AQL of 0.65 for surgical gloves and 1.5 for examination gloves.
3. Understanding the Requirement of EN455-1
The primary requirement of the EN455-1 standard is straightforward: medical gloves for single use should not leak when subjected to the specified water tightness test. This requirement is essential to ensuring the protective function of the gloves.
3.1. What Does ‘Not Leaking’ Entail?
When the standard stipulates that gloves should not leak, it means that there should be no defects or holes that allow water to pass through the glove. Even the smallest hole could compromise the glove’s ability to prevent cross-contamination.
3.2. How Is ‘Not Leaking’ Determined?
The determination of whether a glove leaks or not is based on the water tightness test outlined in the standard. The test involves filling the glove with a specific amount of water and then visually checking for any leakage.
4. The Water Tightness Test: A Closer Look
The water tightness test is central to the EN455-1 standard. Let’s delve into the specifics of this test.
4.1. Referee Testing
Referee testing refers to the specific testing methodology outlined in the standard. It involves using a filling tube of suitable dimensions to fill the glove with water and then inspecting for leaks.
4.2. Routine Testing
Apart from referee testing, the standard also allows for routine testing. This could be another test that has been validated against the water tightness test specified in the standard.
4.3. Test Report
After conducting the test, a test report must be provided. This report should include a reference to the EN455-1 standard, details about the glove, and the test results.
5. Sampling and Inspection: Vital Components of EN455-1
Sampling and inspection are crucial components of the EN455-1 standard as they determine the number of gloves that need to be tested.
5.1. Sampling
The standard prescribes the use of standardized AQL sampling procedures for inspection. This means that a specific number of gloves are randomly selected from a larger batch for testing.
5.2. Inspection Level
The EN455-1 standard uses general inspection level I, but with a minimum sample size and corresponding acceptance/rejection numbers equivalent to sample size code letter L. This ensures that an adequate assessment of the quality of the lot is obtained.
6. Regulatory Implications of EN455-1
The EN455-1 standard carries significant regulatory implications in the realm of medical safety.
6.1. Compliance with EN455-1
Compliance with the EN455-1 standard is a regulatory requirement for manufacturers of medical gloves for single use. It signifies that the gloves have been subjected to rigorous testing and meet the high bar set by the standard for freedom from holes.
6.2. The Role of Regulators
Regulatory bodies play a critical role in enforcing the EN455-1 standard. They conduct audits and inspections to ensure that manufacturers adhere to the standard. They can also take enforcement actions against those who fail to comply.
7. The Broader EN455 Suite
The EN455-1 standard is part of a broader suite of standards known as EN455. This suite covers various aspects of medical gloves for single use.
7.1. EN455-2
EN455-2 sets requirements and testing methods for the physical properties of medical gloves for single use. It ensures that these gloves are durable, flexible, and strong enough to provide effective protection.
7.2. EN455-3
EN455-3 stipulates the requirements and testing methods for the biological evaluation of medical gloves for single use. It ensures that these gloves are safe to use and do not cause adverse biological reactions.
7.3. EN455-4
EN455-4 outlines the requirements and testing methods for establishing the shelf life of medical gloves for single use. It ensures that these gloves maintain their performance characteristics and safety levels over time.
8. The Future of EN455-1
The EN455-1 standard, like all standards, is subject to periodic review and revision to keep pace with technological advancements and evolving industry best practices.
8.1. Potential Revisions
Future revisions of the EN455-1 standard could incorporate new testing methods or technologies that enhance the detection of holes in gloves. They could also refine the requirements based on new insights into glove performance and safety.
8.2. The Role of Stakeholders
Stakeholders, including manufacturers, users, regulators, and standardization bodies, play a crucial role in shaping the future of the EN455-1 standard. Their inputs and feedback are invaluable in ensuring that the standard remains relevant, effective, and beneficial.
9. The Global Relevance of EN455-1
While the EN455-1 standard originates from Europe, it is globally relevant. Many countries and regions outside Europe adopt or reference this standard in their regulatory frameworks for medical gloves.
9.1. Adoption of EN455-1
Countries and regions outside Europe often adopt the EN455-1 standard as part of their regulatory framework for medical gloves. This is a testament to the high regard and trust that the global community places in this standard.
9.2. Global Harmonization
The global adoption of the EN455-1 standard contributes to the harmonization of regulatory requirements for medical gloves. This, in turn, facilitates international trade in these products and ensures a high level of protection for users and patients worldwide.
10. Understanding the Importance of EN455-1
The importance of the EN455-1 standard cannot be overstated. It plays a critical role in ensuring that medical gloves for single use provide reliable protection against cross-contamination.
10.1. Patient Safety
By ensuring that medical gloves do not leak, the EN455-1 standard helps to protect patients from potential cross-contamination during medical procedures. This is a crucial aspect of patient safety.
10.2. User Protection
The EN455-1 standard also protects the users of medical gloves, such as healthcare professionals, by ensuring that the gloves they use are free from holes and, therefore provide reliable protection.
11. Navigating Compliance with EN455-1
For manufacturers of medical gloves, navigating compliance with the EN455-1 standard can be a complex task. However, understanding the standard and its requirements can simplify this process.
11.1. Understanding the Standard
The first step towards compliance with the EN455-1 standard is to understand its requirements. Manufacturers need to familiarize themselves with the standard and its various components, including the terms and definitions, the water tightness test, and the sampling and inspection levels.
11.2. Implementing the Standard
Once they understand the EN455-1 standard, manufacturers need to implement it in their processes. This involves setting up systems and procedures that adhere to the standard’s requirements, training staff on these procedures, and conducting regular audits to ensure ongoing compliance.
12. EN455-1: A Pillar of Medical Safety
In conclusion, the EN455-1 standard is a cornerstone of medical safety. By setting high standards for the quality of medical gloves for single use, it helps to protect both users and patients from cross-contamination during medical procedures. Compliance with this standard is a testament to a product’s quality and reliability, making it a valuable asset for manufacturers and a trusted indicator for users. As our understanding of medical safety evolves, so too will the EN455-1 standard, continuing to play a crucial role in safeguarding health and wellbeing worldwide.
