Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis
This study was published on February 27th 2020 and is one of the first to describe how the IgM-IgG combined anibody test works.
Abstract
The outbreak of the novel coronavirus disease (COVID-19) quickly spread all over China and to more than 20 other countries. Although the virus (SARS-Cov-2) nucleic acid RT-PCR test has become the standard method for diagnosis of SARS-CoV-2 infection, these real-time PCR test kits have many limitations. In addition, high false negative rates were reported. There is an urgent need for an accurate and rapid test method to quickly identify large number of infected patients and asymptomatic carriers to prevent virus transmission and assure timely treatment of patients.
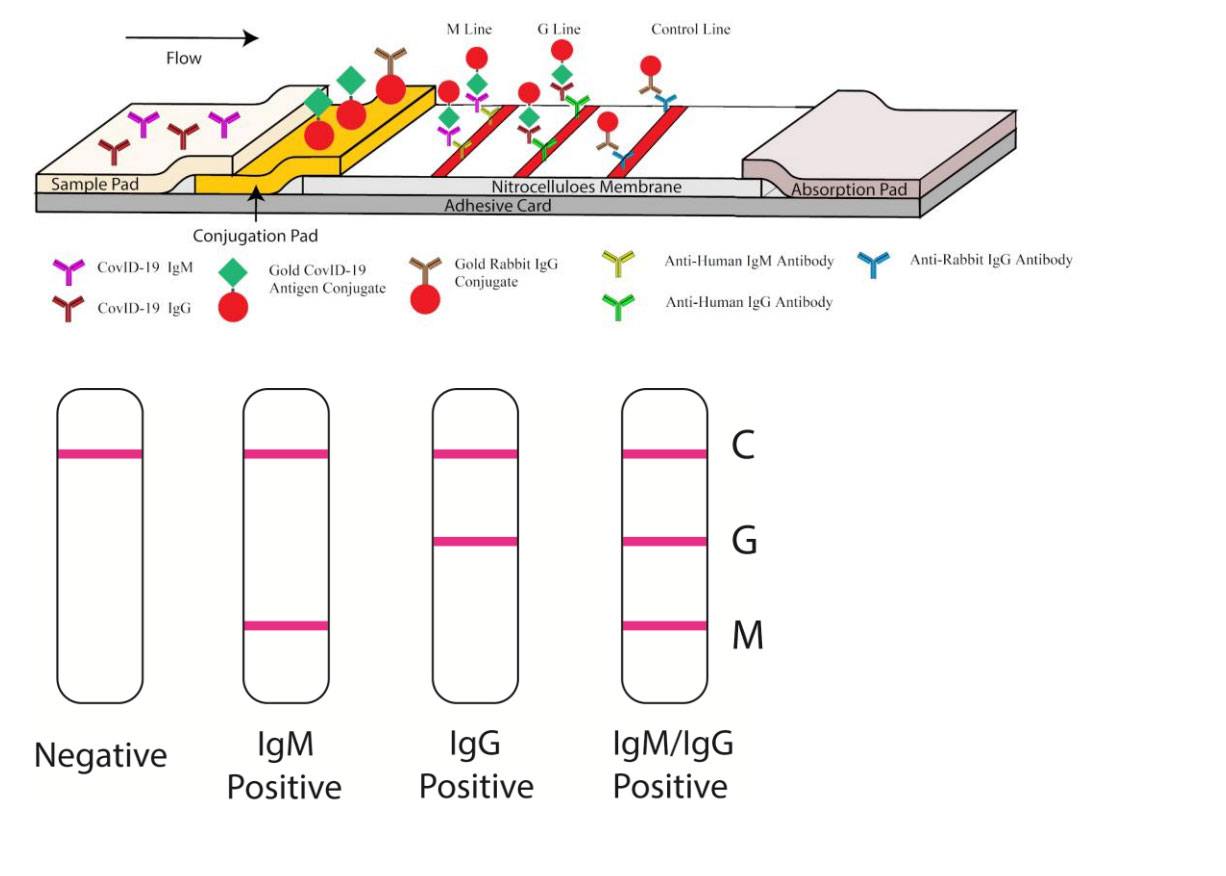
We have developed a rapid and simple point-of-care lateral flow immunoassay which can detect IgM and IgG antibodies simultaneously against SARS-CoV-2 virus in human blood within 15 minutes which can detect patients at different infection stages. With this test kit, we carried out clinical studies to validate its clinical efficacy uses. The clinical detection sensitivity and specificity of this test were measured using blood samples collected from 397 PCR confirmed COVID-19 patients and 128 negative patients at 8 different clinical sites. The overall testing sensitivity was 88.66% and specificity was 90.63%.
In addition, we evaluated clinical diagnosis results obtained from different types of venous and fingerstick blood samples. The results indicated great detection consistency among samples from fingerstick blood, serum and plasma of venous blood. The IgM-IgG combined assay has better utility and sensitivity compared with a single IgM or IgG test. It can be used for the rapid screening of SARS-CoV-2 carriers, symptomatic or asymptomatic, in hospitals, clinics, and test laboratories. This article is protected by copyright.
All rights reserved.